Elast-Eon™ combines the advantageous properties of both polyurethanes and silicone rubbers into a single material, without including the drawbacks of either. It is clinically proven to be a best in class polyurethane for use in long-term implantable and blood contacting medical devices.
Elast-Eon™ polymers are a class of materials suitable for use in long-term implantable medical devices. They are the copolymers of methylene diphenyl diisocyanate (MDI) based polyurethanes and hydroxyl-terminated polydimethyl siloxane, and as a result combine the biostability and biocompatibility of silicones with the mechanical properties of polyurethanes.
Elast-Eon™ polymers outperform all other types of flexible polyurethanes in various biostability and thrombogenicity trials. They are widely accepted as being the most biostable and least thrombogenic of all polyurethane materials. These properties reduce the need for future interventions, improving patient outcomes.
PROPERTIES
Biostable
Non-thrombogenic
Non-calcific
Fatigue resistance
High mechanical performance
Abrasion resistance
FLEXIBILITY / DURABILITY
TENSILE STRENGTH / ABRASION RESISTANCE

Potential Uses
Elast-Eon™ polymers are widely accepted as being one of the most biostable of all polyurethane materials and as such are being used in long-term implantation. Elast-Eon™ may be used in applications including:
- CRM Devices (e.g. Pacemaker)
- Neurostimulation Devices
- Electro Signaling Devices
- Breast Implants
- IUDs
- Implantable Joint Interface Coating
- Stents
- Ear Tubes
- Heart Wall Graft

Processability
Elast-Eon™ is available in a wide range of mechanical properties and is readily processed using standard equipment and techniques including:
- Extrusion
- Dip coating
- Injection moulding
- Spraying
- Electrospinning
- Compression moulding
- Dip casting
- Reaction injection moulding (RIM)
- Vacuum forming
- Heart Wall Graft

CASE STUDY
The development of Elast-Eon™ was originally undertaken in the early 1990’s to address the well documented problems with pacing lead insulation material.
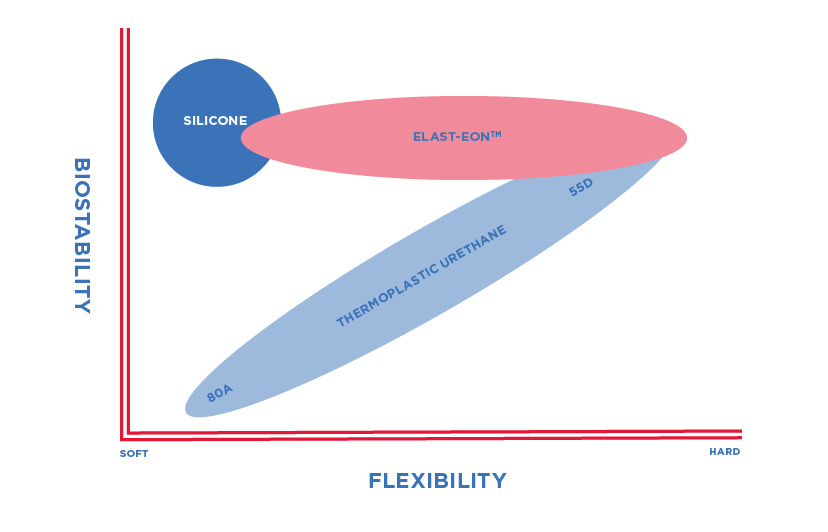
Until the creation of Elast-Eon™, pacing leads had been developed from technology borrowed from other industries such as Silicone Rubber (grades developed for roller pumps) and Polyurethane 80A and 55D (used for undersea telephone cables). Silicone rubber benefits from being soft and flexible with excellent biostability but is susceptible to abrasion. Polyurethane 80A is also softer and flexible with good abrasion resistance but has poor biostability. Polyurethane 55D addresses the biostability problems but is much harder and stiffer. Elast-Eon™ is a silicone-polyurethane co-polymer. The polyurethane content imparts lubricity, strength and abrasion resistance whilst the >40% silicone content imparts flexibility and biostability.
A range of Elast-Eon™ materials is now available with increasing levels of silicone content providing a broad range of mechanical properties, whilst demonstrating the excellent biostability properties of the original invention.
The unique properties of Elast-Eon™ were recognised by Abbott (formerly St Jude Medical) as an opportunity to reduce the incidence of abrasion malfunction of Cardiac Rhythm Management (CRM) leads.
In 2006, under an Elast-Eon™ license, Abbott brought to the CRM market the first novel insulation technology in over 20 years which was marketed as “Optim”.
Elast-Eon™ now features in a broad range of pacing and tachycardia leads with around 8 million implants worldwide. The clinical performance of the Elast-Eon™ leads has been excellent with an 80% reduction in the probability of abrasion related malfunction after 15 years.
Studies on explanted leads have demonstrated Elast-Eon™ to have excellent biostability through 5-year human implant, similar to silicone and the significantly harder 55D grade polyurethane.
The clinical experience of 8 million implants in an extremely harsh environment has demonstrated the benefits of Elast-Eon™ in long term implants and in particular blood contacting devices.